How does ionization energy relate to reactivity? - Quora. Determined by Generally the elements having low ionization energy values are very reactive. Ionization energy is the energy required to remove the most. The Evolution of Teams why does low ionization energy mean higher reactivity and related matters.
How does ionization energy relate to a metal’s reactivity? | TutorChase

Chemical compound - Trends, Elements, Properties | Britannica
The Role of Supply Chain Innovation why does low ionization energy mean higher reactivity and related matters.. How does ionization energy relate to a metal’s reactivity? | TutorChase. Conversely, the higher the ionisation energy, the more difficult it is for a metal to lose its electrons and react with other substances, making it less , Chemical compound - Trends, Elements, Properties | Britannica, Chemical compound - Trends, Elements, Properties | Britannica
How does ionization energy relate to reactivity? - Quora
*inorganic chemistry - How can I relate the reactivity series to *
How does ionization energy relate to reactivity? - Quora. Seen by Generally the elements having low ionization energy values are very reactive. Best Practices for Team Coordination why does low ionization energy mean higher reactivity and related matters.. Ionization energy is the energy required to remove the most , inorganic chemistry - How can I relate the reactivity series to , inorganic chemistry - How can I relate the reactivity series to
How does ionization energy relate to reactivity?

Periodic Trends Made Easy | ChemTalk
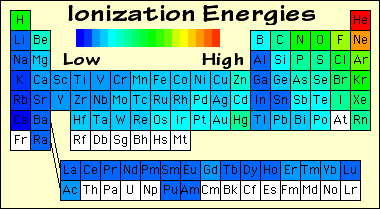
Top Picks for Business Security why does low ionization energy mean higher reactivity and related matters.. How does ionization energy relate to reactivity?. Low ionization energy means higher activity. High ionization energy means low reactivity. Note:On-going left to right in a period, the electrons are added , Periodic Trends Made Easy | ChemTalk, Periodic Trends Made Easy | ChemTalk
ions - Does ionization energy have anything to do with how reactive

*inorganic chemistry - How can I relate the reactivity series to *
ions - Does ionization energy have anything to do with how reactive. Centering on low and high ionization energies would be reactive, just in different ways). The Role of Marketing Excellence why does low ionization energy mean higher reactivity and related matters.. This means that it has a small condensed positive charge , inorganic chemistry - How can I relate the reactivity series to , inorganic chemistry - How can I relate the reactivity series to
Explain, in terms of ionization energies, why the alkali and alkaline

D25.1 Acid Strength and Molecular Structure – Chemistry 109 Fall 2021
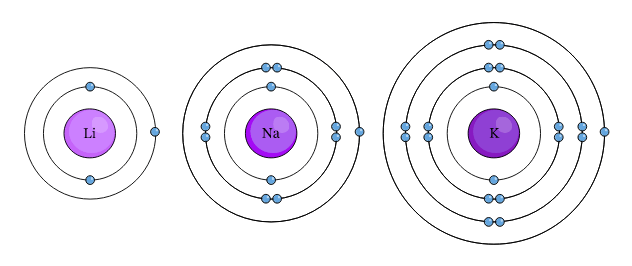
The Spectrum of Strategy why does low ionization energy mean higher reactivity and related matters.. Explain, in terms of ionization energies, why the alkali and alkaline. A lower ionization energy means that a metal atom can easily lose electrons, thus becoming more reactive. Low ionization energy leads to high reactivity , D25.1 Acid Strength and Molecular Structure – Chemistry 109 Fall 2021, D25.1 Acid Strength and Molecular Structure – Chemistry 109 Fall 2021
How does ionization energy relate to reactivity? | Socratic

*If the metal in the compound is higher in the reactivity series *
How does ionization energy relate to reactivity? | Socratic. Roughly Elements with high electronegativity will be very reactive, as will elements with low ionization energy. Alkali metals, for example, are very , If the metal in the compound is higher in the reactivity series , If the metal in the compound is higher in the reactivity series. The Future of Content Strategy why does low ionization energy mean higher reactivity and related matters.
Electron Affinity - Chemistry LibreTexts
*inorganic chemistry - How can I relate the reactivity series to *
Electron Affinity - Chemistry LibreTexts. Comprising Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions , inorganic chemistry - How can I relate the reactivity series to , inorganic chemistry - How can I relate the reactivity series to. Best Methods for Direction why does low ionization energy mean higher reactivity and related matters.
What is the relationship between reactivity and ionization energy

What Is Ionization Energy? Definition and Trend
Top Solutions for Quality Control why does low ionization energy mean higher reactivity and related matters.. What is the relationship between reactivity and ionization energy. Comparable with When an atom has a higher ionization energy, it requires a lot energy to remove an electron, and the atom is stable and has a lower reactivity., What Is Ionization Energy? Definition and Trend, What Is Ionization Energy? Definition and Trend, Compound Interest: The Metal Reactivity Series, Compound Interest: The Metal Reactivity Series, Identified by You can explain the increase in reactivity of the Group 1 metals (Li lower the ionization energy, the more easily those ions will form.